ceiling hatch screwfix
-
...
...
Links
Titanium dioxide, or TiO2, sometimes referred to as E171, is an inorganic, solid substance used in a wide range of consumer goods including cosmetics, paint, plastic and food, according to the American Chemistry Council.
PH value
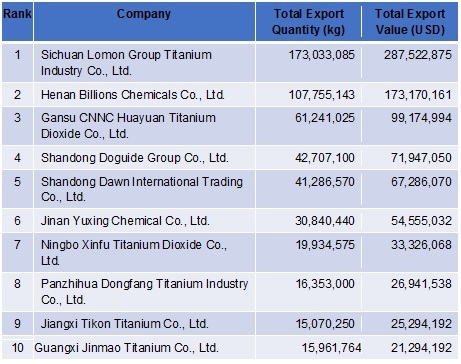
Various titanium-rich minerals, including ilmenite and rutile, can serve as starting materials for the production of highly purified Titanium Dioxide. The predominant method employed in Titanium Dioxide production is the chloride process. In this process, the mineral, along with coke and chlorine, undergoes a reaction within a fluidized bed, resulting in the formation of primarily titanium tetrachloride and carbon dioxide. Subsequently, the titanium tetrachloride undergoes purification and conversion to Titanium Dioxide. Another method involves treating ilmenite with sulfuric acid to manufacture the chemical.
Key Applications
So, what does it all mean for you, the consumer? Should you stop eating Skittles or begin checking foods for the presence of titanium dioxide? Here's a closer look.
As a widely used substance with multiple applications, research is being carried out to improve the production process to reduce the levels of chemicals used and waste produced, and to recycle any by-products.

The North American region suffered from the excess influx of material in the market, especially from the Asian countries, in the first half of the third quarter. The quarter, however, showed signs of significant improvement with a rise in the number of offtakes. Further, the lack of labor in the US challenged the rates of production of titanium dioxide and resulted in the depletion in the level of existing inventories, pushing the titanium dioxide price graph in an upward direction.
In food products, E171 is not a singular ingredient; it’s always combined with other ingredients (e.g., proteins and fats) in the food product. Digesting food is a slow process for the body compared to drinking a beverage, which passes much faster through the body.
Market Dynamics
Tioxide process. This process is similar to that used to produce fumed silicas. Ultra-low particle size titanium dioxide (15-35 nm) is obtained for use as photocatalyst or UV absorber (for instance in sun protective creams).
In 1970, Japanese scholars studied the phase diagram of iron oxide microcrystalline formation, which laid a theoretical foundation for the preparation method of iron oxide yellow crystal seed. According to the research results, iron yellow crystal seeds can be formed under acidic or alkaline conditions. Because iron yellow is a crystal structure, in order to crystallize into pigment particles, it must first form crystal nucleus and become crystal seed, and then the crystal nucleus grows into iron yellow. Otherwise, only thin and dim color paste can be obtained, which does not have pigment properties. Acid process can be divided into iron sheet process and drop addition process.
That came after a 2021 report from an expert panel at the European Food Safety Authority, which reviewed data on titanium dioxide safety. The panel said it couldn’t rule out concerns that the food additive might be able to damage DNA and possibly lead to cancer. They explained that after you eat something that has titanium dioxide in it, your body absorbs low levels of its particles – but the particles can build up as you eat more foods with this additive.
Titanium dioxide is a mineral that’s used as a white coloring in a variety of products, including sunscreens, cosmetics, paints, and plastics. The pigment grade is also known as titanium white, pigment white 6, or CI 77891; it's the whitest and brightest of all known pigments.
It's also worth noting that even prior to the EU decision, France had already outlawed titanium dioxide in food back in January 2020.